Tech Advantages
A PATENTED ULTRASONIC TRANSDERMAL DELIVERY TECHNOLOGY
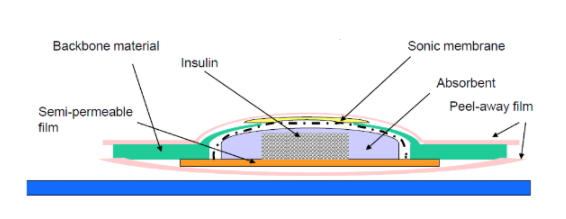
Unlike conventional patches used in medicine today, U-STRIPTM transdermal drug delivery system opens the door as a new tool for investigating the delivery of both small and large compounds through the skin using pulsed ultrasound to first dilate the skin pores and then push the drug into the dermis.
Drug Formulation
Transdermal Specialties Global (TSG) is offering R&D services to those firms who desire a faster pathway to FDA approval, from as long as 12 years of drug development to as little as 4 years through a transdermal pathway. The new transdermal Laboratory is designed to develop new applications for ultrasonic transdermal drug delivery. Already the company is working upon a Parkinson’s Patch, a Patch for Multiple Sclerosis, and a Migraine patch. Through a series of Research Collaborations, the company is expanding the number of drugs which can be delivered via U-STRIPTM technology from the 20 or so products currently on the market, to over 175 pharmaceutical preparations on the Company’s target list.
- First step is to determine what effects if any ultrasound has on target drug compound and evaluate drug/device interaction
- Patch Is Loaded with the Target Drug and the Liberation Rate Determined at Differing U/S Frequencies and for Different Absorbent Pads
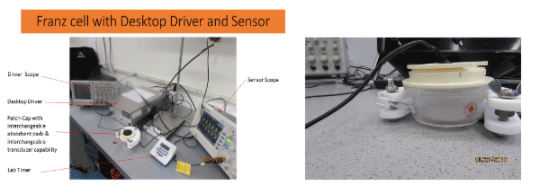
- Franz cell Studies determine delivery rate at various U/S settings
- Conduct rapid screening and characterization of raw materials
- Human Skin Irritation study both short & Long term
Drug Evaluations
STEP DESCRIPTION NOTES TIMELINE 1 Ultrasound vs Compound Goal to be sure the ultrasound does not damage the compound. HPLC analysis. 2-4 weeks 2 Liberation Test Franz cell Studies determine delivery rate at various U/S settings 3 Human pK Demonstration 5 subject human delivery test, pK values 12-20 weeks
R&D
Advantages
TSG’s first product the Trans-Insulin™ Patch is now in phase-3 clinical trials. Our formulation experts, analytical chemists, and clinical and regulatory specialists are leaders in both science and drug development
Transdermal may dramatically reduce the time to market for a particular compound. Average time for patch approval is 4 years.
PATCH PRODUCTION CAPACITY: 1MM UNIT/MONTH
To guarantee your product meets global compliance standards, our commercial manufacturing process combines extensive knowledge of device and drug quality systems requirements with pharmaceutical manufacturing expertise, including:
- Establishing a quality agreement with client
- Patch Production Capacity: 1 MM Units/Month
- Maintaining process performance
- Assuring continued compliance with ever-evolving regulatory standards
- Managing supply chain with respect to API, component sourcing, and packaging