DRUG FORMULATION
Transdermal Specialties is offering R&D services to those firms who desire a faster pathway to FDA approval, from as long as 12 years of drug development to as little as 4 years through a transdermal pathway. The new transdermal Laboratory is designed to develop new applications for ultrasonic transdermal drug delivery. Already the company is working upon a Parkinson’s Patch, a Patch for Multiple Sclerosis, and a Migraine patch. Through a series of Research Collaborations, the company is expanding the number of drugs which can be delivered via U-STRIPTM technology from the 20 or so products currently on the market, to over 175 pharmaceutical preparations on the Company’s target list.
TSI has the expertise and experience in finding creative solutions for taking your drug and developing a stable, viable formulation.
First step is to determine what effects if any ultrasound has on target drug compound and evaluate drug/device interaction
Patch Is Loaded with the Target Drug and the Liberation Rate Determined at Differing U/S Frequencies and for Different Absorbent Pads
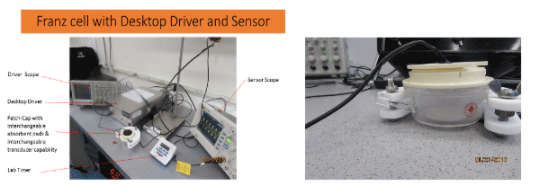
Franz cell Studies determine delivery rate at various U/S settings
Conduct rapid screening and characterization of raw materials
Human Skin Irritation study both short & Long term
DRUG EVALUATION
STEP DESCRIPTION NOTES TIMELINE
| 1 | Ultrasound vs. Compound | Goal to be sure the ultrasound does not damage the compound. HPLC analysis. | 2-4 weeks |
| 2 | Liberation Test | Franz cell Studies determine delivery rate at various U/S settings | |
| 3 | Human pK Demonstration | 5 subject human delivery test, pK values | 12-20 weeks |